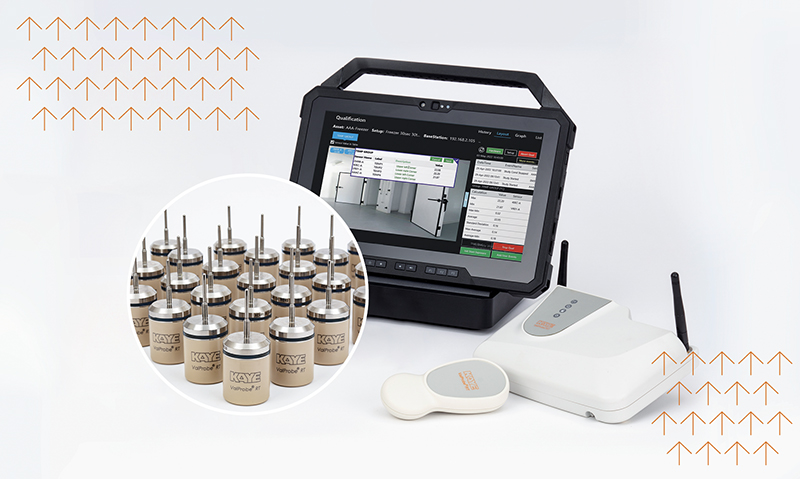
Thermal Validation Systems - Wired and Wireless
The Kaye product range is relied upon by the world’s leading pharmaceutical and biotechnology companies to validate and monitor critical assets and processes like sterilization as required by governing regulatory bodies.

Kaye LabWatch® IoT - Advanced Cloud Monitoring with Intelligent Sensor Communication
Kaye LabWatch IoT powered by FCX is an intelligent Cloud based monitoring system designed for Life Sciences, to continuously monitor, alarm and collect critical GxP data for analysis, reporting and real-time actionable knowledge.
Our Locations
Get in Touch with Us !
Find your direct and local support. Reach out to our Kaye team or our authorized partner in your region for your sales enquiries, technical or services support. We look forward to assisting you with all your thermal validation system, environmental monitoring, and cold chain needs.
Latest Products
-
Kaye ValProbe RT CO2 Logger
The 3-in-1 data logger from the Kaye ValProbe RT product line offers the capability to measure critical parameters such as CO2 (carbon dioxide), relative humidity (RH), and temperature with a single device. For applications where these measurements are quality-critical, the Kaye ValProbe RT CO2 Logger provides economical, accurate, and GxP-compliant recording and documentation of these parameters.
-
Kaye ValProbe RT 1.3
ValProbe RT, Kaye’s industry leading wireless validation system, is ready to operate at version 1.3. This major software update implements many new features that greatly improve user experience.
-
Common Reporting Tool 1.4
The Common Reporting Tool is a comprehensive software compatible with Windows based PCs. It enables you to create reports from your Kaye Validator AVS or ValProbe RT Qualification study files, offering a practical solution for documenting the results of your validation studies.
-
Kaye LTR-200
The Kaye LTR-200 is a multi-purpose Temperature Calibrator specifically designed to address the capacity and flexibility needs for thermal validation. This essential Temperature Calibrator utilizes its ability to perform Temperature Calibration on up to 24 thermocouples capacity. Due to hybrid technology, the LTR-200 Temperature Calibrator saves hours of time and effort when calibrating or verifying validation Temperature Sensors.
-
Kaye ValProbe RT 5-Channel Bendable Logger
The real-time Kaye ValProbe RT data loggers, obtainable in one and two sensor versions, are now also available with five sensors in conjunction with bendable probes. The advanced electronics of the loggers in combination with the PT-1000 Sensors achieves an accuracy of up to 0.10°C and at the same time enable an extended battery life. This Logger supports up to 100,000 samples per measurement channel – so a total of up to half a million datapoints per device.
News
-
Jan 28, 2025

Green Energy Initiative: Kaye Europe Center of Excellence Now Powered by 20% Solar Energy
At Kaye, a subsidiary of Amphenol, we are excited to share a significant update in our commitment to sustainability and renewable energy. With the installation of a 40 kVA solar system at our Pforzheim location in Germany, we will be able to cover more than 20% of our annual electricity consumption exclusively with self-generated solar power in the future.
-
Jan 16, 2025
Hardware and Software Upgrades for Kaye ValProbe RT Enhance User-Friendliness and Expand Application Scope
The latest expansion of the Kaye ValProbe RT® product line introduces additional technical and user-oriented features that improve user-friendliness and broaden the application spectrum for GxP compliant validation studies. Thanks to improved hardware and software features, such as the support for up to 50 Kaye ValProbe RT® real-time data loggers per study, application-centric applications such as CO2 incubator qualification are sustainably supported. An additional standard evaluation tool based on AFNOR FDX15-140 also simplifies the qualification of climatic chambers of any type and size.
-
Jan 08, 2025

Risk Analysis in Pharmaceutical Manufacturing: The Importance of Qualifying Critical Process Equipment
The pharmaceutical industry constantly faces the challenge of meeting stringent regulatory requirements and high-quality standards. One of the essential tasks is the qualification of critical process equipment. Traditionally, predefined guidelines and standards are followed. However, the increasing focus on quality and safety necessitates more: a comprehensive risk analysis that identifies and evaluates potential sources of hazards. In this paper, we highlight why risk analysis is an indispensable part of the qualification process and how it can be effectively integrated into the qualification plan. The goal is to raise awareness and underscore the importance of risk analysis with subsequent risk evaluation.
-
Dec 11, 2024

Ensuring Safe Transport and Optimizing On-Site Data Logger Handling
In the meticulous environment of pharmaceutical operations, ensuring the integrity and security of data loggers is paramount. The management of Kaye ValProbe (RT) data loggers requires high-quality, robust solutions to simplify transport, storage, and management—key aspects to maintain the accuracy of crucial data.
-
Dec 03, 2024

Kaye's Time Warp: Providing Comprehensive Support
Everyone who has ever purchased a measuring system knows this. The true quality of the supplier is not only shown in printed words on a data sheet or a favorable offer. It shows itself when it comes to essential after-sales support. Be it the annual calibration, help, and support during commissioning, training for changing operating personnel, or bridging bottlenecks in your own measuring device. Particularly attentive readers of our blog series are certainly looking forward to what Kaye has had to offer in this area from the beginning.