Kaye Solutions for Environmental Monitoring
Application at a Glance
Continuous monitoring and regular validation of various application areas such as storage environments, cold rooms, LN2 tanks, tissue banks, and more are fundamental components of a GMP-compliant quality system. Critical parameters such as temperature and relative humidity are essential components of the necessary documentation to ensure the integrity and safety of products in these environments. Other important critical process parameters like pressure or CO2 expand the spectrum of parameters to be measured depending on the application.
The Importance of Continuous Monitoring and Validation
In various industries, especially in the pharmaceutical and biotechnological sectors, continuous monitoring of environmental parameters is crucial and therefore regulated by guidelines and standards specific to the GxP environment. This includes not only monitoring the parameters but also setting up alarm systems that notify when critical thresholds are reached. This allows for immediate intervention to prevent potential damage to stored goods and ensure compliance with regulatory standards such as ISO and GMP.
Critical Environmental Parameters (Depending on the Application):
- Temperature: Essential for maintaining the integrity of temperature-sensitive products such as vaccines.
- Relative Humidity: Critical for preventing the deterioration of products due to moisture. Keyword: Stability tests according to ICH.
- Pressure/Differential Pressure: Important in personnel or material airlocks in cleanroom environments to ensure the purity and integrity of the surroundings.
- Light Intensity: Monitoring light intensity to ensure compliance with specific requirements, especially in laboratories and production facilities.
- Door Contacts: Recording the status of doors to ensure critical environments are properly sealed and protected from external influences.
- CO2: Important in incubators and other controlled environments to ensure an optimal atmospheric composition.
- And others
Depending on the specific application, further parameters can be monitored. Essentially, any values that can directly or indirectly affect the quality of the final product.
Monitoring these parameters is crucial to ensure the prescribed conditions for each application and to guarantee GMP-compliant quality assurance. Kaye offers a variety of sensors combined with a recognized continuous monitoring and alarm system that is known for its reliability and meets all regulatory requirements.
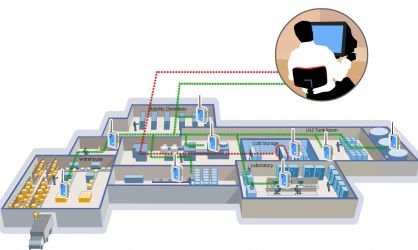
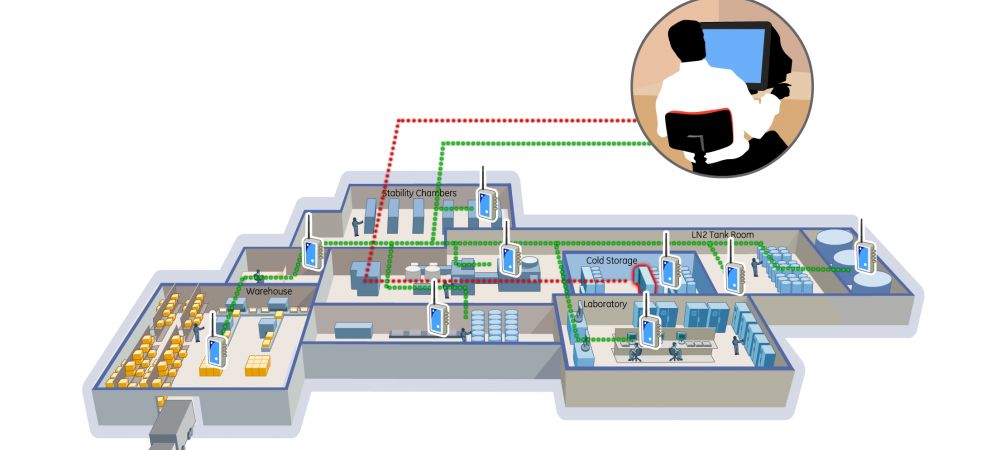
Applications for Continuous Monitoring Systems
- Warehouses: Ensuring the specified storage conditions.
- Repositories: Protecting valuable and sensitive materials.
- Blood/Tissue Banks: Maintaining the viability of biological samples.
- Laboratories: Ensuring accurate and reliable experimental conditions.
- Cold Rooms: Storing temperature-sensitive semi-finished and finished products in a constant temperature environment.
- Stability Chambers: Monitoring the conditions crucial for stability studies.
- LN2 Tanks: Ensuring extremely low temperatures required for certain biological samples.
- Animal Rooms: Providing a controlled environment for laboratory animals.
- Pharmacies: Ensuring the safety and efficacy of stored medications.
- And more
Advantages of Kaye's Continuous Monitoring Systems
- Regulatory Compliance: Adheres to all current standards and regulations in the GxP environment.
- Real-Time Data: Immediate access to current environmental conditions.
- Alarm Systems: Instant notifications when predefined thresholds are exceeded/subceeded.
- Data Logging and Reporting: Comprehensive documentation for regulatory compliance.
- High-Precision Sensors: Ensuring accurate measurements of critical parameters.
- Scalability: Solutions that can be tailored to any size of operation.
- Data Security and Data Integrity: Ensured by GxP-validated software and firmware.
- Unrestricted Data Access: Through integration into a GxP-compliant AWS Cloud.
- Hardware/Software/Service: Everything from a single source and directly from the manufacturer. We support your project from the planning phase to IQ/OQ/PQ and the regular calibration of the Kaye sensors used, including re-qualification of the entire monitoring system.
- Continuous Development: Both hardware and software to comply with changing directives and regulations.
Continuous Monitoring of Critical Process Parameters
Continuous monitoring of critical process parameters is an indispensable part of the validation of these processes. Sensors and data loggers continuously capture the process parameters, and this data is archived as part of the quality documentation. This not only ensures compliance with legal requirements but also guarantees that products remain in optimal condition at all times.
Kaye's Technological Solutions
Kaye offers the Kaye LabWatch IoT, a GxP-compliant solution for continuous monitoring systems. These state-of-the-art monitoring technologies ensure process security and high operational efficiency, ultimately improving the quality and integrity of pharmaceutical products.
Contact Us
If you have any questions, contact us directly or get in touch with one of our certified dealers in your country.