Kaye Solutions for Liquid-Nitrogen (LN2)
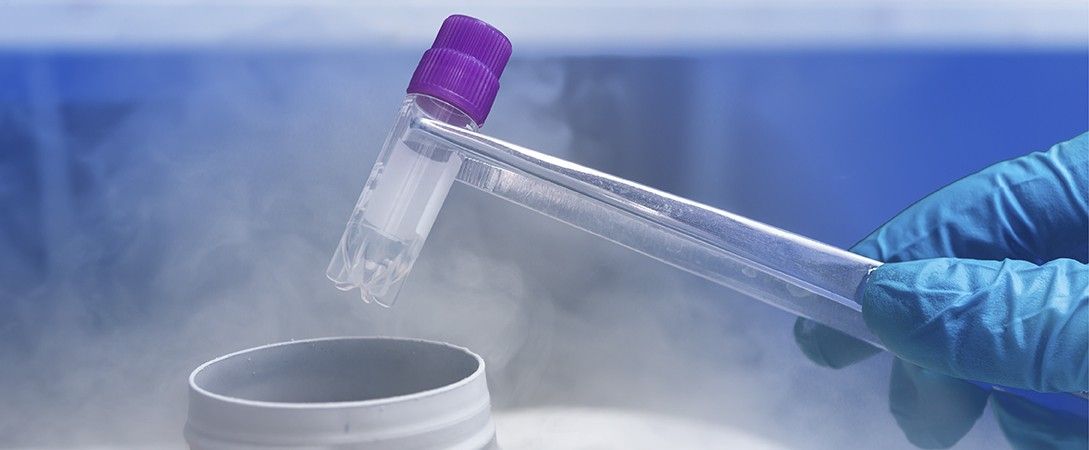
Cryogenic gases like liquid nitrogen (LN2) are crucial in the pharmaceutical and biotechnology industries, enabling the safe storage of intermediates and raw materials by preventing interactions with moisture or oxygen. LN2 and dry ice are also used in various processes, including mixing, storage, and cryogenic cooling.
Benefits of Cryogenic Containers
Cryogenic containers are essential in these industries, allowing the storage of biological samples (cells and tissues) at extremely low temperatures, ensuring their integrity for future studies. The EU-GDP requires these containers to be qualified and validated to ensure pharmaceutical quality during distribution.
Need for Qualification and Validation
At atmospheric pressure, LN2 has a constant temperature of -196°C. Since its boiling point depends on ambient pressure, it's important to qualify both the nitrogen and the cryogenic container used.
Sample Storage in the Gas Phase of LN2
Samples are preferably stored in the gas phase of LN2 to minimize cross-contamination (as samples are not in direct contact with liquid nitrogen), enhance safety in case of leaks or spills, and maintain stable temperatures (typically below -150°C), suitable for most biological samples.
Maintenance and Monitoring
Gas phase storage requires careful monitoring and maintenance of cryogenic containers to ensure constant temperatures and adequate nitrogen levels. Temperature mapping studies help identify and document variations, ensuring uniform temperature distribution.
Solutions for Temperature Validation
For -196°C calibration, we offer the Kaye AVS Validator with the Kaye LN2 Comparator. This setup enables semi-automatic calibrations and documentation, using LN2's boiling point for precise measurements, minimizing its evaporation rate for stability over several hours.
Visit our website for more information on our products and solutions. We provide tailored solutions for pharmaceutical and biotechnology needs.