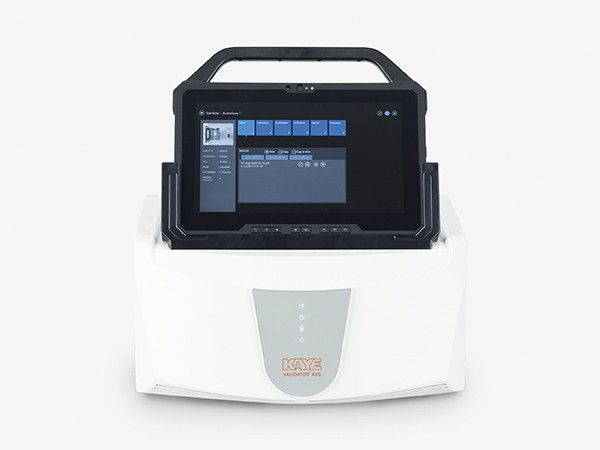



Kaye Validator AVS - Autoclave Temperature Mapping - Temperature Validation System
Benchmark Temperature Validation System with patented Asset Centric Data Management Features - a new flexible approach to Validation.
Description
Advanced Validation Technology
The Kaye Validator AVS (Advanced Validation System) is an all-in-one temperature mapping and thermal validation system that combines precise sensor measurements with all GxP requirements for calibration and traceability to national standards, while generating compliant reports and managing the validated assets and validation equipment. With its temperature measurement capabilities, the Validator AVS takes accuracy to a whole new level, offering improved hardware features, a user-friendly data handling concept, and an intuitive software interface making an Autoclave Temperature Mapping easy and compliant.
Reducing Your Cost of Ownership
With our integrated temperature validation and temperature monitoring system, the Kaye Validator AVS reduces your cost of ownership by simplifying the entire thermal mapping and validation process, bearing testament to a better and swifter approach towards ensuring maximum performance while performing an Autoclave Temperature Mapping study but offering significant advantages for any kind of Thermal Mapping exercises of any critical assets.
Lifting Validation to the Next Level
Introducing the Kaye Validator AVS, a game-changer that is set to take validation to the next level with its core thermal mapping functions to perform professional autoclave validation, Autoclave Temperature Mapping and thermal validation studies of many other critical processes in the pharmaceutical and biotech industry.
The Kaye Validator AVS introduces a completely new validation concept. The AVS console will be the common platform for all validation activities. It is dedicated for validation tasks only – so no worries about operating systems. The Software is preloaded – with a customized interface for validation equipment, simplifying the validation process
Our AVS console serves as a dedicated platform for all validation tasks like collecting the sterilization temperature. The AVS Software eliminates worries about operating systems. We've made ease our standard, with preloaded software highlighted by a customized interface aimed at streamlining the validation process.
Autoclave Temperature Mapping Study
A commitment to ensuring product quality and regulatory requirements has led us on a path to innovate advanced validation technology that introduces an accurate temperature mapping solution for your autoclave. By investing in Kaye's AVS Validator, precise temperature mapping and measurement data is captured at multiple locations throughout the autoclave. Real-time visualization of the autoclave's temperature distribution becomes seamless with our AVS software. Kaye's solution not only demonstrates compliance but enhances process control, thereby ensuring consistent product quality.
Common Reporting Tool Software
The Kaye Validator AVS works splendidly with our Common Reporting Tool that allows you to conveniently conduct post analysis of your validation studies even from the comfort of your office on a separate PC. Generate your report of your Autoclave Temperature Mapping wherever you are, on-site or in your office desk.
Download a fully functional 30 days trial version here!
Features
Validator AVS Wired Temperature Validation System
- Enhanced data handling and redundancy (4-levels)
- Expanded input capacity, types, and range
- Improved scan time 1/sec for 48 inputs (EN554/ISO17665)
- Stand-alone operation with Console docking station
Validator AVS Console
- Hardened, rugged Console pre-loaded with all Kaye software
- Easy-to-operate / state-of-the art intuitive user interface
- Ethernet/ WiFi/ Docking Station connections
- Asset centric concept for process equipment and Kaye hardware
- Console capable of running existing Kaye product software.
- Improved Reporting Analysis Tool –backward compatible
- Simplified validation (GAMP-5)
Applications
The Temperature Validation System is built for many applications, including
- Steam Sterilizers (Autoclaves)
- Dry Heat Sterilizers
- Steam in Place (SIP)
- Water Cascade/Fall Sterilizers
- Incubators
- Stability Chambers
- Freezers
- Freeze Dryer/Lyophilisation
- Vessels
Specifications
Technical specifications
| Analog Input | Up to 48 |
|---|---|
| Thermocouples | Type T, J, K,E,B,R,N,S: 0.1°C; T+ limited range 0.01°C resolution |
| Scanning Speed | 48 channels per second |
| Internal Memory | 4 gb for data collection |
| Input Impedance | 10KΩ. Source greater than 10KΩ produces open circuit indication |
| Common Mode Rejection | 160 db (8 inputs/sec) @ line frequency 145 db (12 inputs/sec) @ line frequency 140 db @ DC |
| Max. Common Mode Voltage | 100V pk ch-to-ch350V pk ch-to ch to frame ground |
| Normal Mode Rejection | 82 db @ 60 Hz (8 inputs/sec)69 db @ 60 Hz (12 inputs/sec) |
| Voltage Input | 0 to 10 VDC |
| Resolution | 1:72,000 |
| Voltage Input Accuracy | 30 days: ±(0.003% of reading + 2 counts + 4 microvolts) 1 year: ±(0.006% of reading + 2 counts + 4 microvolts) |
| Sensitivity | 0.5 microvolts/count on most sensitive range |
| Voltage Temp. Coef. | ±(0.1 microvolts + 0.001% reading)/°C |
| Compensator Temp. Coef. | ±0.01°C per °C |
| Input Terminal Temperature Non-uniformity |
±0.1°C from calibrated terminal |
| Input Ranges | -6 to 30mV, -12 to 60mV, -60 to 300mV, -2 to 10V |
| Environmental | Temperature: 0 to 50°C (32 to 122°F) Relative humidity: 95% non-condensing |
| Power | 90 to 250 VAC, 50/60 Hz |
| Fuse Rating | 4A Slow Blow |
| Size | 190H X 411W X 381 mm D (457 mm with SIM) 7.5 in H x 16.2 in W x 15 in D (18 in with SIM) |
| Weight | 10.60 kg (23.4 lbs) |
| Battery | Lithium ion with minimum 3 hours of battery backup |
Documents
English
Portuguese
Italian
Spanish
French
Japanese
Video


