Cryo containers are found in various applications in the pharmaceutical and biotechnology industries. Be it to store biological samples like cells and tissues at extremely low temperatures to preserve them for future studies to use, or even for transportation. Thus, cryo containers are part of the cold chain and therefore must be qualified and validated according to EU GDP (European Union Good Distribution Practice). These guidelines were developed to ensure the quality of pharmaceuticals throughout the entire distribution process from manufacturing to consumption.
The Corona pandemic has affected the world in many ways, including an increased need for cryo processes. The development and distribution of COVID-19 vaccines have amplified the demand for ultra-cold storage solutions, as some vaccines need to be stored at extremely low temperatures. Additionally, the pandemic has led to increased research and development in the field of biotechnology, which has further increased the need for cryo solutions for the storage and transport of biological, and sometimes extremely temperature-sensitive samples.
But why do cryo containers need to be qualified and validated? Purely physically, for example, liquid nitrogen at atmospheric pressure has a stable and even temperature of -196°C.
Obviously, liquid nitrogen changes its boiling temperature depending on the environmental pressure. The qualification requirement, therefore, does not affect the liquid nitrogen but the combination with the used cryo container.
In the pharmaceutical and biotechnology industries, the samples brought in for storage are preferably stored in the gas phase of liquid nitrogen, especially when storing biological samples.
The advantages of storing in the gas phase compared to direct storage in liquid nitrogen are:
1. Minimization of cross-contamination: Storage in the gas phase helps minimize the risk of cross-contamination between samples, as the samples are not directly in liquid nitrogen.
2. Safety: Storage in the gas phase is safer, as the samples are less likely to be damaged in the event of a leak or spill of the container.
3. Temperature: The temperature in the gas phase is slightly higher than in liquid nitrogen but remains extremely low (usually below -150°C), which is sufficient for most biological samples.
It is important to note that careful monitoring and maintenance of the container are required when storing in the gas phase of liquid nitrogen to ensure that the nitrogen level does not drop too low, and the temperature remains constant.
Ideally, the temperature in the gas phase of the liquid nitrogen should be almost homogeneous. However, temperature differences can occur, depending on the exact construction and insulation, as well as the location of the container. And it is these potentially possible temperature fluctuations that must be determined and documented by a temperature mapping (distribution study). It is always advisable to monitor the temperature at several points in the gas phase and confirm it in a validation protocol to ensure the necessary temperature homogeneity.
Practical solution
Kaye Validator AVS in combination with the Kaye LN2 Comparator - Liquid Nitrogen Bath
The perfect solution for calibrations at -196°C
As with any temperature validation study, the simple 4-step principle applies to the validation of cryo containers:
- Calibration and adjustment of the used sensors based on the given required measurement uncertainties
- Qualification - Determination of temperature values at different measuring points (distribution study)
- Post-calibration to ensure that the used temperature sensors still work within the given measurement uncertainty
- Documentation of the determined calibration and measurement results
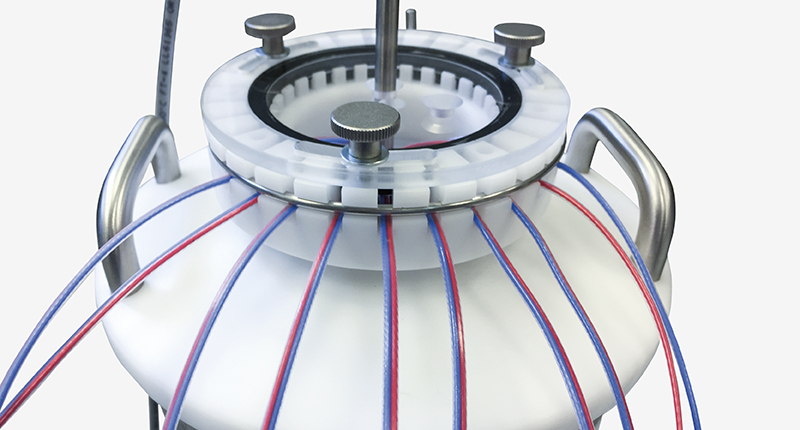
The Liquid Nitrogen (LN2) Comparator is an easy-to-use device for calibrations that require extremely low temperatures. Taking advantage of the boiling point of liquid nitrogen, this comparator in combination with the Kaye Validator AVS allows for semi-automatic calibrations and adjustment of the used temperature sensors at -196°C. Of course, the automatic creation of all calibration and qualification documents is also part of the solution.
With the included thermocouple holder, calibrations can be carried out on up to 48 thermocouples simultaneously. In addition, it ensures easy and safe handling of the thermocouples, as well as the reference temperature probe necessary for traceable calibration (Kaye IRTD-400).
The LN2 Comparator was designed to keep the evaporation rate of the liquid nitrogen as low as possible and offers you the possibility to perform calibrations under stable conditions for several hours at a time.
To enquire about a product or service, you can reach out to us online and one of our representatives will be happy to assist you! To contact us, please visit us here: https://www.kayeinstruments.com/en/contact
To request a demo of any of our products, please visit our demo request website here: https://www.kayeinstruments.com/en/demo
Follow us on LinkedIn or register for our newsletter: https://www.kayeinstruments.com/de/newsletter-subscription
Copyright: Amphenol Corporation