Warehouse Monitoring Solutions - Warehouse Mapping
Warehouse mapping is defined by regulatory guidelines as a seasonal requirement to ensure that storage conditions are maintained at defined specifications. Performing this crucial operation relies significantly on Warehouse Operations and calls for robust systems such as the Storage Mapping process. This is a very critical step in ensuring your products and assets are kept safe and within the threshold of standards while stored in your facility.
That’s why it’s important to perform a warehouse mapping study using equipment that is built to withstand and detect any type of temperature change inside your facility. This aspect of warehouse operations and storage mapping is key in upholding product integrity. These temperature changes usually occur during large swings in exterior temperature. Even though the internal temperature change may be minuscule, it could lead to threatening changes in temperature-sensitive materials.
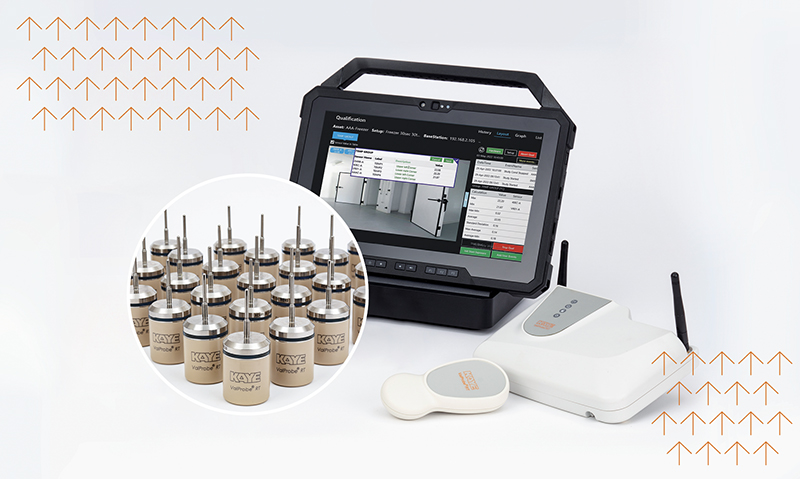
At least twice per year, usually once in the winter and once in the summer, it’s important to protect your temperature and humidity-sensitive products by performing a thermal warehouse mapping of your storage, chambers, freezers, refrigerators, or warehouse by using a Kaye RF ValProbe®II temperature data logger. Warehouses should utilize storage mapping to keep track of product placements and monitor environmental conditions. Oftentimes, the heat of the summer months and frigid cold of the winter can change the temperature and conditions of your storage facility enough to impair or even damage products. Scheduling a routine, bi-annual warehouse mapping is one of the best ways to prevent issues with your GMP systems.
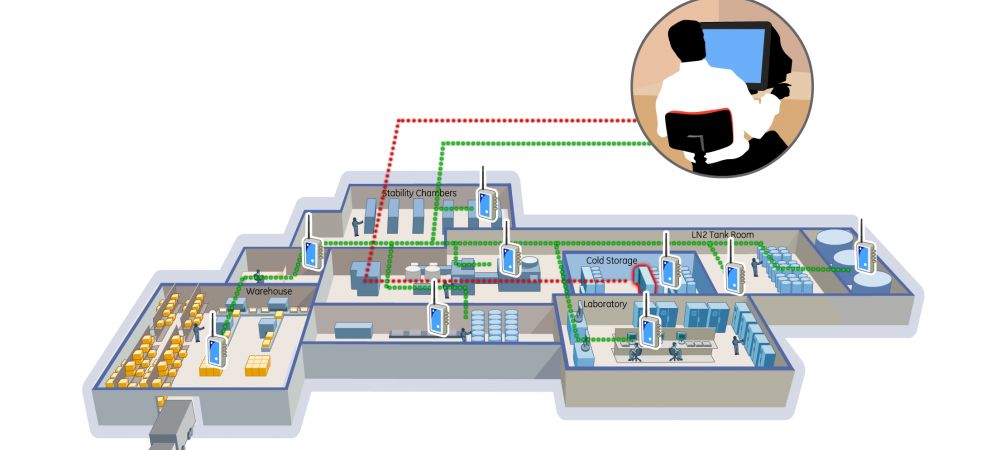
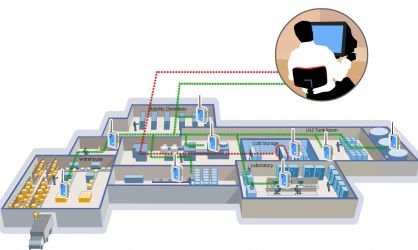
When preparing for a warehouse mapping, there are many factors you must first think about. It’s vital that you plan ahead to determine the number of studies you’ll be doing, the length of the study, acceptable temperature for safe and allowable operation, quantity and types of sensors needed and the locations of the sensors. Additionally, you will need to have a good understanding of the size of your facility, temperature swings, hot spots or heat envelope locations, as well as airflow of both storage and foot traffic areas.
Then it is important to have a proper procedure ready to execute the mapping and analyzing the results. Using the right equipment can give users a drastic advantage when performing a thermal mapping. The hardware being used is important, and as equally important is the software. What good is the data if you aren’t able to read it easily and interpret what it’s telling you? That’s where the Kaye RF ValProbe II system is a standout.
The Kaye RF ValProbe's will continuously monitor your facility's temperature and give you an instant readout, making it simple for you to understand your site's conditions. The new ValProbe RF II data loggers that Kaye created is a significant upgrade from our earlier ValProbe RF I. The Kaye RF ValProbe II wireless data logger integrates breakthrough RF Mesh technology with enhanced technology for Thermal Validation.
During a warehouse mapping, the probes can be easily placed throughout the facility and read using our intuitive software on any PC or smartphone. Learn more about our warehouse mapping solutions by visiting the Kaye RF ValProbe®II page. When you’re ready for a demonstration or if you have questions, don’t hesitate to reach out to one of our expert account managers.
If you’re not ready to purchase equipment yet, view our rental solutions here to find which products will work best when doing a warehouse mapping of your facility.
Knowledge Library
Challenges/Practices
Norms/Guidelines
Articles/White Papers